41 h2y acid or base
PSW | PDF - Scribd psw.txt - Free ebook download as Text File (.txt), PDF File (.pdf) or read book online for free. acid base - Neutralization reactions - Chemistry Stack Exchange If the acid is H X 2 Y or H X 3 Z, the neutralization reactions are. 2 N H X 3 + H X 2 Y ( N H X 4) X 2 Y. 3 N H X 3 + H X 3 Z ( N H X 4) X 3 Z. It never produces H X 2 O. If one atom H from N H X 3 is replaced by an organic radical R, a primary amine R N H X 2 is obtained and the neutralization reaction produces also an ammonium salt R N H X 3 X.
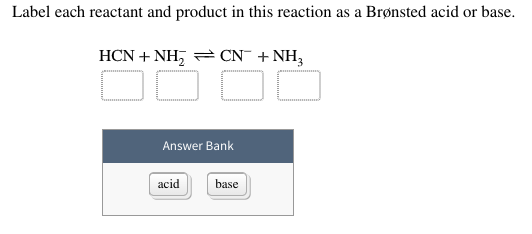
Label each reactant and product in this reaction as a bronsted acid or ... Label each reactant and product in this reaction as a bronsted acid or base. h2y- + h2z- < ===> h3y + hz2-Answers: 2 Show answers : ) Another question on Chemistry. Chemistry, 22.06.2019 05:30. Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? ...

H2y acid or base
Acid and Base Ionization Constants | Chemistry for Non-Majors ... In general, the stronger the acid, the weaker its conjugate base. Likewise, the weaker the acid, the stronger its conjugate base. Strong acids are 100% ionized in solution. Weak acids are only slightly ionized. Phosphoric acid is stronger than acetic acid and so is ionized to a greater extent. Acetic acid is stronger than carbonic acid, and so on. (PDF) Química Analítica, 6ta Edicion - Academia.edu Química Analítica, 6ta Edicion - Gary D. Christian Our New Approach to Reporting - SEC Our shareholder base is widely diversified, with approximately 39 per cent of shares held in Australia, 30 per cent in Europe, 18 per cent in North America, 8 per cent in South Africa and 5 per cent in Asia. Globally, the Company spent in the order of US$12.5 billion sustaining its businesses.
H2y acid or base. Acid-Base Equilibrium Part 1: How to Use the pKa Table Thus, once an acid loses the proton, it becomes a conjugate base.And once a base accepts a proton, it becomes a conjugate acid.Remember, that when we are talking about the conjugates we are always talking about the products of a specific acid-base reaction. The conjugate base that we made in the reaction above (HSO 4 -) can dissociate further and be an acid in a different reaction. Solved Identify each reactant and product in this reaction - Chegg This problem has been solved! See the answer Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y−+H2Z−↽−−⇀H3Y+HZ2−H2Y−+H2Z−↽−−⇀H3Y+HZ2− Expert Answer 95% (57 ratings) Given reaction is H2Y- + H2Z- -------> H3Y + HZ2- As we know pr … View the full answer Previous question Next question PSW | PDF - Scribd psw.txt - Free ebook download as Text File (.txt), PDF File (.pdf) or read book online for free. What is the conjugate base of acid H2S ? | Yeah Chemistry Conjugate acid-base. whats an easy way to memorize the tests for cations. How to draw this in condensed structural formula? What would happen to the caffeine if the sublimation were performed at atmospheric pressure . How many liters of O2 gas, measured at 781 mmHg and 24 ∘C , are required to completely react with 2.7 mol of Al ? ...
Tips for Conjugate Acid and Base Formulas - Concept That's a short cut for going from an acid to it's conjugate base. Now on the flip side, base to conjugate Acid. Say if I have a base let's say I have Ammonia or NH3 and that's on water so we use our double yield sign. Water is acting like an acid in this case. Water is going to donate a proton to the NH3. Ethylenediaminetetraacetic acid - Wikipedia Ethylenediaminetetraacetic acid (EDTA) is an aminopolycarboxylic acid with the formula [CH 2 N(CH 2 CO 2 H) 2] 2.This white, water-soluble solid is widely used to bind to iron and calcium ions. It binds these ions as a hexadentate ("six-toothed") chelating agent.EDTA is produced as several salts, notably disodium EDTA, sodium calcium edetate, and tetrasodium EDTA. Answered: Identify each reactant and product in… | bartleby Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y−+H2Z−↽−−⇀H3Y+HZ2−. DOC 1970 - chemmybear.com The equations for two acid-base reactions are given above. Each of these reactions proceeds essentially to completion to the right when carried out in aqueous solution. (a) Give the Bronsted-Lowry definition of an acid and a base. ... The graph below shows the results obtained by titrating a different weak acid, H2Y, with the standardized ...
Chem 106 - Sapling Questions - Topic 3 Flashcards | Quizlet Acids and Bases Chem 106 - Sapling Questions - Topic 3 STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Live Label each reactant and product in this reaction as a Brønsted acid or base. H2Y- + H2Z- <-> H3Y + H3-2 Click card to see definition 👆 Conjugate pairs H2Y- base-acid H3Y H2Z- acid-base H3-2 Click again to see term 👆 1/5 Previous PDF Frontier Central School District / Overview Created Date: 3/5/2013 2:44:24 PM acid base - How to predict the color of a pH indicator using Le ... In the presence of N a O A c, a weak base, H X 2 Y is completely deprotonated, thus no red colour is observed. In addition, a part of H Y X − is deprotonated too: the second equilibrium isn't completely on the left side. Some amount of Y X 2 − is present too. As a result, the green colour (yellow + blue) is observed. 35241 PDFs | Review articles in LEWIS ACIDS Any chemical species which accepts an electron-pair from a LEWIS BASE in a chemical bonding reaction. | Explore the latest full-text research PDFs, articles, conference papers, preprints and more ...
Which triel bond is stronger? TrHX⋯H2Y versus TrH2X⋯H2Y (Tr = Ga, In; X ... The H 2 Y molecules own negative MEP regions around Y atom. The most negative electrostatic potential value ( Vmin) becomes larger with the order of S < O, which is in consistent with the electronegativity of Y atom (3.44 for O, 2.58 for S), and H 2 O molecule is a stronger Lewis base [ 37 ].
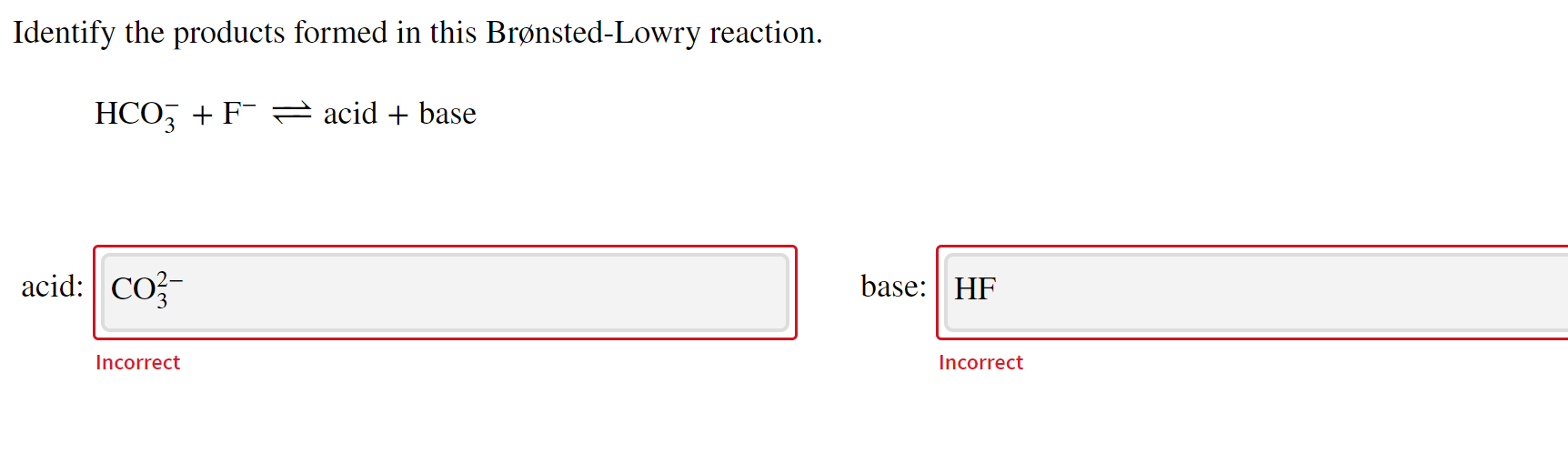
Identify each reactant and product in this reaction as a Brønsted acid ... conjugate acid of CO2−3: conjugate acid of NH3: Give the formula of the conjugate base of HSO−4. conjugate base: Give the formula for the conjugate acid of HSO−4. conjugate acid: What is the hydrogen ion concentration of a solution with pH=11.25? dentify the products formed in this Brønsted-Lowry reaction. HSO−4+HBrO↽−−⇀acid+base
Chem 121 Exam 3 Flashcards | Quizlet Classify each reactant and product in this reaction as an acid or base according to the Brøsted theory HF, H2O, F-, H3O+ ... H2Y-: base H2Z-: acid H3Y: acid HZ2-: base. Identify the conjugate acid for each base. - conjugate acid of HSO−4: - conjugate acid of SO−4^2-- conjugate acid of NH3. H2SO4 HSO4-NH4+ Identify the pair of species that ...
Our New Approach to Reporting - SEC Our shareholder base is widely diversified, with approximately 39 per cent of shares held in Australia, 30 per cent in Europe, 18 per cent in North America, 8 per cent in South Africa and 5 per cent in Asia. ... the emissions of sulphuric acid mist have been significantly reduced. A preventive approach to managing our health issues will focus ...
Is HI (Hydroiodic acid) a Strong or Weak Acid - YouTube There are two ways to determine whether HI is a strong or weak acid. The first it to memorize the seven common strong acids. For general chemistry courses t...
3.0 M HI Unknown NaOH initial burette reading 0.2 ml 0.7 ml final ... 3.0 M HI Unknown NaOH initial burette reading 0.2 ml 0.7 ml final burette reading 47.6 ml 37.5 ml What is the concentration of the base (NaOH) in the - 4193013
Label each reactant and product in this reaction as a Bronsted acid or ... Label each reactant and product in this reaction as a Bronsted acid or base. H2Y- + H2Z- <===> H3Y + HZ2- Posted one year ago. Q: 31) Which Is The Correct Lewis Structure For Magnesium Bromide? A) B) Mg:Br Mg:Br: D) 32) Assign Formal Charges To Cach Atom In The Resonance Structures Of N:0.
Is Hydrofluoric Acid (HF) a Strong or Weak Acid? - ThoughtCo Hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. As the concentration of hydrofluoric acid approaches 100 percent, it's acidity increases because of homoassociation, where a base and conjugate acid form a bond: 3 HF ⇆ H 2 F + + HF 2-
(Solved) - Label each reactant and product in this reaction as a ... According to the Bronsted-Lowery ...



Post a Comment for "41 h2y acid or base"